Health
Mirena Contraceptive License Extended to Eight Years of Use
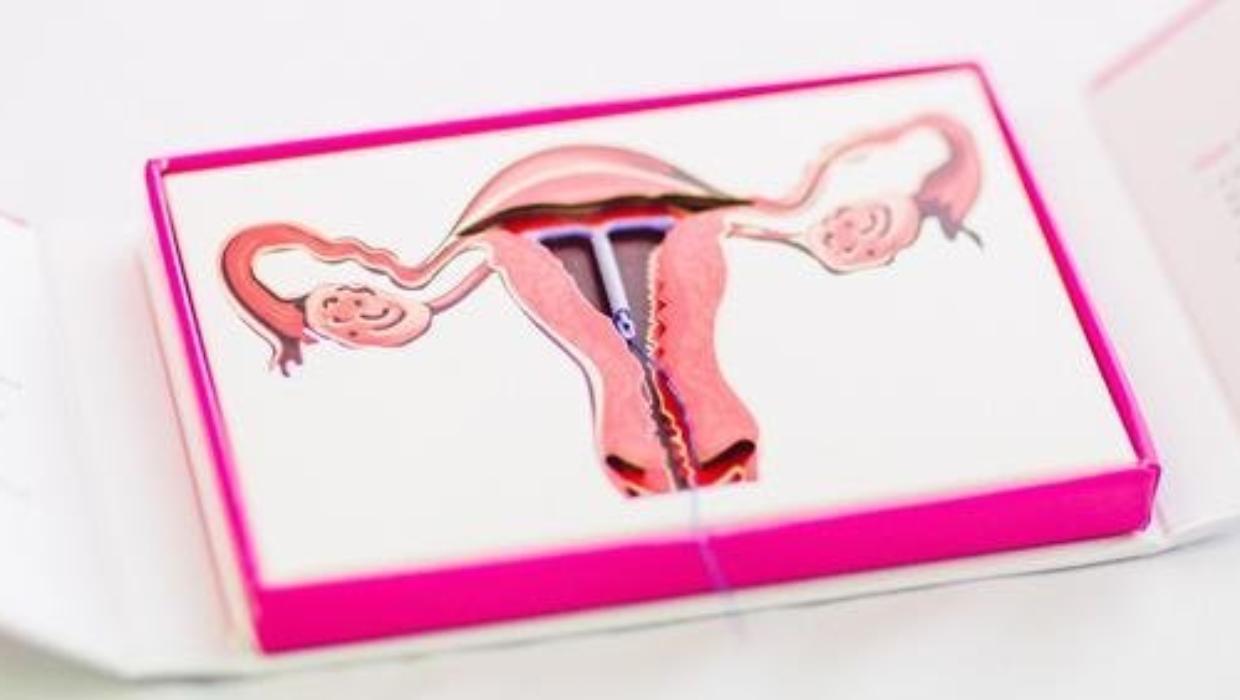
The U.S. Food and Drug Administration (FDA) has officially extended the license for the Mirena contraceptive device, allowing its use for up to eight years. This decision is significant for many individuals seeking reliable contraceptive options. The Mirena, a T-shaped plastic device, releases 52 mg of levonorgestrel, a hormone widely used in various contraceptive pills.
The FDA’s approval was announced on October 17, 2023, marking a pivotal moment for reproductive health. Previously, the device was approved for a five-year usage period. This extension offers users an enhanced option for long-term birth control, reducing the need for frequent replacements.
Impact on Women’s Health Choices
The decision to extend the Mirena’s license is expected to have a broad impact on women’s health choices. By allowing a longer duration of use, it addresses the needs of those who prefer a hassle-free contraceptive method. The hormone released by the device not only prevents pregnancy but also aids in managing heavy menstrual bleeding, providing dual benefits for many users.
Healthcare professionals are optimistic about the extension. Dr. Sarah Thompson, a gynecologist, stated, “This extension allows patients to have more control over their reproductive health without the constant concern of needing to replace their contraceptive method regularly.” This perspective highlights the importance of accessible and effective contraceptive solutions in women’s healthcare.
Regulatory Approval Process
The FDA’s approval process involved extensive research and clinical trials to ensure the safety and efficacy of the Mirena for an extended period. According to the agency, the decision was based on data demonstrating that the device remains effective and safe for use beyond the initial five years.
The move aligns with ongoing efforts to enhance reproductive health services and options available to individuals. As contraceptive methods evolve, the healthcare community continues to advocate for more comprehensive choices that meet diverse needs.
In conclusion, the extension of the Mirena contraceptive license to eight years represents a significant advancement in reproductive health care. This decision not only provides users with a longer-lasting option but also emphasizes the importance of accessible contraceptive methods in managing individual health needs.
-

 World2 weeks ago
World2 weeks agoPrivate Funeral Held for Dean Field and His Three Children
-

 Top Stories2 weeks ago
Top Stories2 weeks agoFuneral Planned for Field Siblings After Tragic House Fire
-

 Sports3 months ago
Sports3 months agoNetball New Zealand Stands Down Dame Noeline Taurua for Series
-

 Entertainment3 months ago
Entertainment3 months agoTributes Pour In for Lachlan Rofe, Reality Star, Dead at 47
-

 Entertainment2 months ago
Entertainment2 months agoNew ‘Maverick’ Chaser Joins Beat the Chasers Season Finale
-

 Sports3 months ago
Sports3 months agoSilver Ferns Legend Laura Langman Criticizes Team’s Attitude
-

 Sports1 month ago
Sports1 month agoEli Katoa Rushed to Hospital After Sideline Incident During Match
-

 World3 weeks ago
World3 weeks agoInvestigation Underway in Tragic Sanson House Fire Involving Family
-

 Politics2 months ago
Politics2 months agoNetball NZ Calls for Respect Amid Dame Taurua’s Standoff
-

 Top Stories2 weeks ago
Top Stories2 weeks agoShock and Grief Follow Tragic Family Deaths in New Zealand
-

 Entertainment3 months ago
Entertainment3 months agoKhloe Kardashian Embraces Innovative Stem Cell Therapy in Mexico
-

 World4 months ago
World4 months agoPolice Arrest Multiple Individuals During Funeral for Zain Taikato-Fox


















